Introduction to HTA Regulation
The Health Technology Assessment (HTA) Regulation (2021/2282) represents a significant milestone towards a unified approach in evaluating health technologies across the European Union. This regulation, in force since January 12, 2022 and applicable from January 12, 2025, aims to harmonize the assessment of health technologies, ensuring that patients have access to effective and safe treatments. The development of health technologies is crucial for economic growth and innovation within the EU and is essential for safeguarding public health.
What is Health technology assessment (HTA) and Joint Clinical Assessment (JCA)?
Health technology assessment (HTA) is a scientific evidence-based process that allows to determine the relative effectiveness of new or existing health technologies. HTA focuses specifically on the added value of a health technology in comparison with other new or existing health technologies.
HTA involves a systematic, transparent, impartial, and robust approach that considers clinical, economic, social, and organizational aspects.
One of the core components of the HTA Regulation is the Joint Clinical Assessment (JCA). The JCA provides a comparative analysis of clinical evidence on a health technology against other existing technologies or procedures. By standardizing the clinical evaluation process, the JCA seeks to reduce redundancy and ensure consistency across EU member states, ultimately promoting more efficient and equitable healthcare decision-making. This assessment focuses solely on clinical effectiveness and safety, excluding the domains of HTA of the cost and economic evaluation of a health technology, and the ethical, organisational, social and legal aspects related to its use, which remain under the jurisdiction of individual EU member states.
The implementation of the JCA will be phased in over the next few years:
- 12 January 2025: Oncology medicinal products and Advanced Therapy Medicinal Products (ATMPs)
- 13 January 2028: Medicinal products with orphan designation
- 13 January 2030: All centrally authorized medicinal products
Stakeholder Involvement in HTA
The success of the HTA process heavily relies on the involvement of various stakeholders, including health technology developers (HTDs), healthcare professionals, patient organizations, and EU member states’ HTA bodies. The HTD is responsible for submitting comprehensive information and evidence for the JCA, while the Coordination Group, comprising representatives from member states, oversees the HTA process. This group ensures methodological guidelines, promotes cooperation, and maintains transparency.
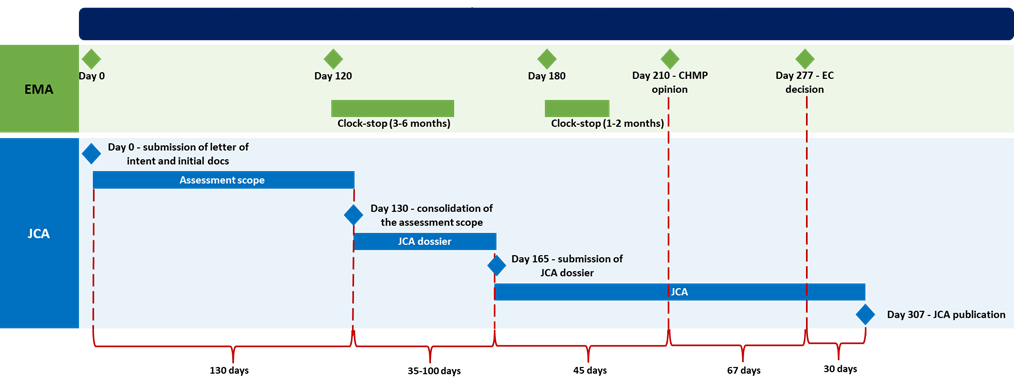
Timeline of the Joint Clinical Assessment (JCA) Process
The JCA process begins parallel to the EMA’s centralized procedure and develops according to the following steps and timelines:
- Initiation:
- Day 0: HTD submits the letter of intent and initial documentation to EMA and HTA Secretariat.
- Assessment Scope:
- Day 1- Day 130: Assessment scope is defined using the PICO framework (Population, Intervention, Comparator, Outcome).
- Day 130: the JCA subgroup consolidates the assessment scope, which is shared with the HTD.
- JCA Dossier Preparation:
- Day 130: HTD begins preparing the JCA dossier.
- Day 165: Submission of JCA dossier by HTD. The deadline for submission is within 100 days from receiving the PICO and 45 days before the expected date for the CHMP opinion.
- Evaluation:
- Day 165- Day 277: The JCA subgroup reviews and drafts the JCA and summary reports. The JCA Subgroup shall finalise the revised draft joint clinical assessment and summary reports at the latest on the date of the adoption of the EU Commission decision granting the marketing authorisation and submit them to the Coordination Group for endorsement.
- Day 210: CHMP opinion.
- Day 277: European Commission’s decision granting the marketing authorization.
- Finalization and Publication:
This structured timeline ensures a comprehensive and coordinated evaluation process, enabling timely and effective decision-making for health technologies across the EU.
Implementing Acts and Consultation Feedback
The HTA Regulation includes several implementing acts:
- First Implementing Act: Related to JCA for medicinal products, officially adopted on May 23, 2024.
- Second Implementing Act: Addressing conflict of interest management, made available as draft, and submitted for public consultation, with adoption scheduled for July 2024.
- Third Implementing Act: Concerning cooperation by exchange of information with the European Medicines Agency (EMA), under public consultation until July 24, 2024.
- Fourth to Sixth Implementing Acts: Expected by the end of 2024, these acts will focus on:
- Joint scientific consultation for medicinal products
- Joint scientific consultation for medical devices
- Joint clinical assessment for medical devices
Conclusion and Future Implications
The HTA Regulation fosters solidarity and coordination among EU member states, promoting high-quality health technology assessments. By implementing these joint assessments, the EU aims to streamline access to effective treatments, reduce inequalities, and enhance overall public health. While the JCA results are not binding, they significantly influence national decisions, contributing to a more harmonized and efficient healthcare system across Europe.
Regulatory Pharma Net remains committed to monitoring regulatory developments and providing insights to help stakeholders navigate the evolving landscape of health technology assessments. Our team is ready and available to support Pharmaceutical Companies with the anticipation of the possible PICOs, the development of JCA and national P&R dossiers, to streamline health technology assessments and ensure timely market access.
For further details, updates and support, please contact us at info@regulatorypharmanet.com and follow us on LinkedIn.